GALVANIC CORROSION
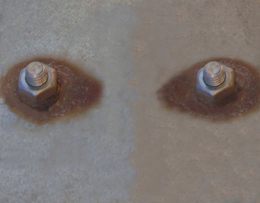 Galvanic corrosion is produced when two different electrochemical activity metals are in contact between them. In this case, exists a difference in electric potential between this metals in such a way that the current flow rusts one metal of the pair formed.
Galvanic corrosion is produced when two different electrochemical activity metals are in contact between them. In this case, exists a difference in electric potential between this metals in such a way that the current flow rusts one metal of the pair formed.
Metals different between them act one as ánodo and the other one as cátodo. The bigger the potential difference between metals, the bigger is the probability that galvanic corrosion appears, only in one of the metals.
Galvanic attack can be uniform or located in the alloy unions, depending on the conditions. Galvanic corrosion can be particularly severe when protective layers from corrosion do not appear or are erased by erosion. To reduce this kind of corrosion, rust protective films can be applied, as well as isolation of one metal from the other one.
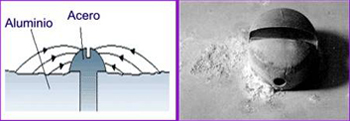 This type of corrosion has to be taken in account when choosing a solution based in coatings for belleville washers, depending on their application and length expected. Pressure and friction generated in these areas can be high due to strengths between disc springs are applied in really narrow areas. Depending on the coating type, its duration can be affected.
This type of corrosion has to be taken in account when choosing a solution based in coatings for belleville washers, depending on their application and length expected. Pressure and friction generated in these areas can be high due to strengths between disc springs are applied in really narrow areas. Depending on the coating type, its duration can be affected.
Regarding of coatings with Nickel base, if coating damage is produced, these ones result with a bigger corrosion due to a high potential difference in the electrochemical serie.
